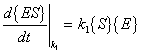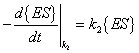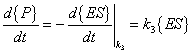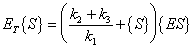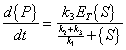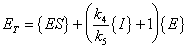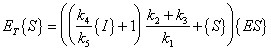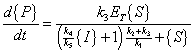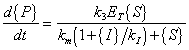Before describing the particular implementations of the various types of kinetics, it
is necessary to review the theory behind Monod kinetics. Monod kinetics are different
from, but still based upon Michaelis-Menten kinetics for enzymes. One can think of Monod
Kinetics as describing a chain of enzymatically mediated reactions with a limiting step
described by Michaelis-Menten kinetics. This is why the equations for both kinetic models
are identical. The following paragraphs describe the development and theory behind
Michaelis-Menten kinetics.
The basic assumption behind Michaelis-Menten Enzyme kinetics is that enzymes catalyze
reactions by first forming an enzyme-substrate complex (Grady and Lim, 1975). This
substrate complex will either decay back to enzyme and substrate (the reverse of the
previously mentioned reaction) or irreversibly decay to enzyme and product. These chemical
reactions for complex formation and product formation respectively are:
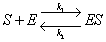 |
(A.1) |
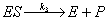 |
(A.2) |
| Where: |
S = substrate |
|
E = enzyme |
|
ES = enzyme-substrate complex |
|
P = product |
|
k1 = rate constant for complex formation |
|
k2 = rate constant for reverse complex formation |
|
k3 = rate constant for product formation |
The rates for the above reactions would be as follows:
| Where: |
k1 = rate constant for complex formation |
|
k2 = rate constant for complex reverse formation |
|
k3 = rate constant for product formation |
|
{S} = concentration of substrate |
|
{E} = concentration of free enzyme |
|
{ES} = concentration of substrate-enzyme complex |
|
{P} = concentration of product concentration |
Furthermore, it is assumed the above set of equations are in equilibrium such that:
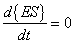 |
(A.6) |
Therefore:
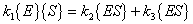 |
(A.7) |
A mass balance on the total enzyme is given as:
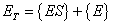 |
(A.8) |
Combining equations (A.7) and (A.8) and substituting into equation (A.5) gives:
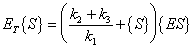 |
(A.9) |
and
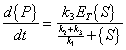 |
(A.10) |
so let
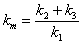 |
(A.11) |
therefore
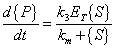 |
(A.12) |
| Where: |
{P} = concentration of product |
|
{ES} = concentration of enzyme-substrate complex |
|
{S} = concentration of substrate |
|
ET = total complexed and un-complexed enzyme |
|
k1 = rate constant for complex formation |
|
k2 = rate constant for reverse complex formation |
|
k3 = rate constant for product formation |
|
km = "half-saturation" concentration |
Which is analogous to Monod kinetics, k3 is analogous to the maximum
specific substrate utilization rate, ET is analogous to biomass
concentration, and km is analogous to the half saturation constant.
Monod kinetics and its variations, along with other bio-kinetic equations will be
presented in the following discussion.
In competitive inhibition an inhibitory complex can combine with the controlling enzyme
in addition to the reaction equation (A.1). This additional complex prohibits the enzyme
from forming the complex with the substrate of interest.
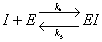 |
(A.13) |
| Where: |
I = inhibitor |
|
E = enzyme |
|
EI = enzyme-substrate complex |
|
P = product |
|
k4 = rate constant for complex formation |
|
k5 = rate constant for reverse complex formation |
Equation (A.8) now looks like:
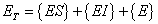 |
(A.14) |
| Where: |
ET = total complexed and un-complexed enzyme |
|
{E} = concentration of free enzyme |
|
{ES} = concentration of substrate-enzyme complex |
|
{EI} = concentration of inhibitor-enzyme complex |
After substitution of equation (A.13) (with the assumption of equilibrium) equation
(A.14) becomes:
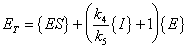 |
(A.15) |
The same derivation for Michaelis-Menten Kinetics as presented above applies:
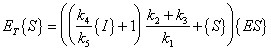 |
(A.16) |
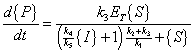 |
(A.17) |
so let
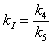 |
(A.18) |
therefore
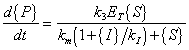 |
(A.19) |
| Where: |
{P} = concentration of product |
|
{ES} = concentration of enzyme-substrate complex |
|
{S} = concentration of substrate |
|
{I} = concentration of inhibitor |
|
ET = total complexed and un-complexed enzyme |
|
k1 = rate constant for complex formation |
|
k2 = rate constant for reverse complex formation |
|
k3 = rate constant for product formation |
|
km = "half-saturation" concentration |
|
kI = "saturation" constant for inhibitor |
There are other types of inhibition, such as un-competitive, and substrate inhibition
which are not presented here.
Semprini (1991) studied the competitive inhibition of TCE degradation by methane. A
double Monod form of inhibition kinetics was used:
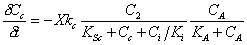 |
(A.20) |
| Where: |
Cc = concentration of contaminant |
|
Ci = concentration of the inhibitor |
|
CA = concentration of the electron acceptor |
|
KSc = saturation constant for contaminant |
|
KA = saturation constant for the electron acceptor |
|
kc = maximum transformation rate |
|
Ki = inhibition constant |
It should be noted equation (A.20) is in the form of double Monod kinetics, however,
the first term in the equation is the same form as equation (A.19). The second order
electron acceptor term was included since the presence of an electron acceptor was
required for the contaminant transformation.