| Chapter 6. Editing an Aquifer System |
To edit the rate or rule data for a reaction, the user must bring up the rate data editing page for the reaction. This is the same regardless of the reaction type. First the Form View Reactions Window must be active. Then select the rate data tab. BUGS SCRATCHPAD supports many different types of reactions. Each reaction appears in a different screen. This section illustrates how to enter rate or rule data for each type of a Reaction.
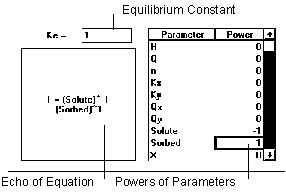
Figure 6.11 Equilibrium Reaction
The equilibrium reaction adjusts the values of parameters given in the Stoichiometry relationship (if such adjustment does not cause a parameter to have a negative concentration value) such that the ratios of their values match the equilibrium constant.
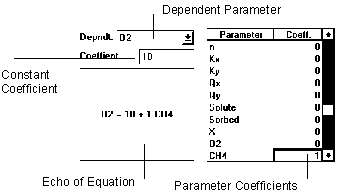
Figure 6.12 Equality Reaction
The equality reaction simply sets the dependent parameter to the value specified by the linear combination of parameters and constant.
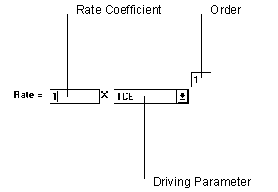
Figure 6.13 Single Order Reaction
The single order reaction only uses data from one parameter to determine the rate of reaction.
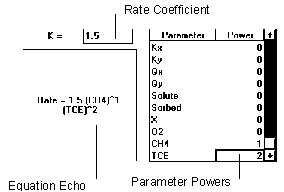
Figure 6.14 Mixed Ordered Reaction
The mixed order reaction uses values of multiple parameters to determine the rate of the reaction.
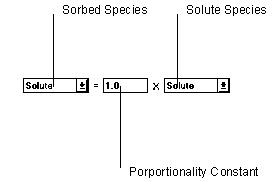
Figure 6.15 Linear Equilibrium Isotherm
The linear equilibrium sorption isotherm adjusts parameters using the relationship defined in the Stoichiometry to set the left hand side of the equation equal to the right hand side.
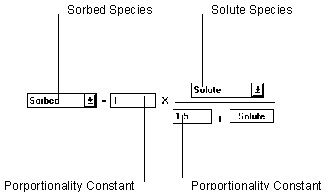
Figure 6.16 Langmuir Equilibrium Isotherm
The Langmuir Equilibrium sorption isotherm adjusts parameters using the relationship defined in the Stoichiometry to set the left hand side of the equation equal to the right hand side.
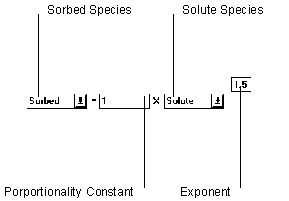
Figure 6.17 Fredulich Equilibrium Isotherm
The Fredulich Equilibrium sorption isotherm adjusts parameters using the relationship defined in the Stoichiometry to set the left hand side of the equation equal to the right hand side.
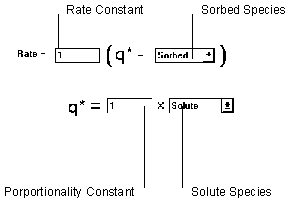
Figure 6.18 Linear Kinetic Isotherm
The linear kinetic isotherm computes a target sorbed phase concentration with the linear isotherm relationship. A mass transfer equation is then used to determine the rate of reaction.

Figure 6.19 Langmuir Kinetic Isotherm
The Langmuir Kinetic isotherm uses the Langmuir isotherm relationship to compute a target sorbed phase concentration. A mass transfer equation is then used to determine the rate of reaction.
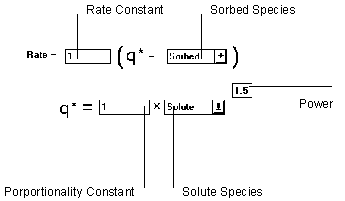
Figure 6.20 Fredulich Kinetic Isotherm
The Fredulich Kinetic isotherm uses the Fredulich isotherm relationship to compute a target sorbed phase concentration. A mass transfer equation is then used to determine the rate of reaction.
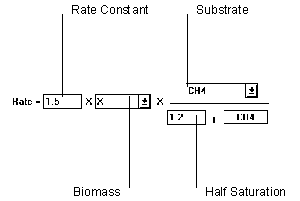
Figure 6.21 Single Monod Reaction
The Single Monod equation relies on values from two parameters to determine the rate of reaction. However, the biomass portion can be ignored by setting to "unity".
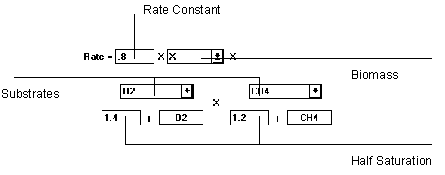
Figure 6.22 Double Monod Reaction
The Double Monod equation relies on three parameters to compute the rate of reaction, two substrate and one biomass. If the biomass component is not needed, it can be set to "unity".
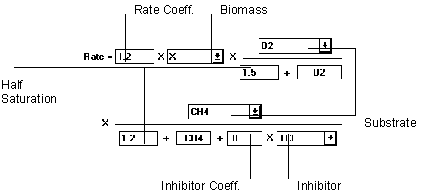
Figure 6.23 Competitive Inhibition Reaction
The Competitive Inhibition equation includes a term for a competitive parameter in one of the substrate terms of a double monod relationship. If the biomass term is not needed, it can be set to unity.
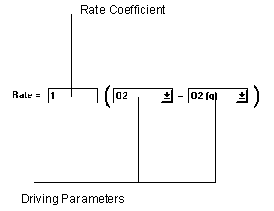
Figure 6.24 Mass Transfer Reaction
The mass transfer equation simply uses the difference between two parameter values to compute the rate of reaction.

Figure 6.25 Rule Curve Reaction
The Rule Curve reaction can be used if all of the above reactions were not appropriate. In addition to accessing the values of parameters, this reaction can also use time as input. For example, an injection schedule could be entered. Independent variables (x) must be entered in acceding order. BUGS SCRATCHPAD uses a simple lookup routine to find values in the list.
| User's Manual | Contents | Next |
� Copyright 1998, BUGBYTES, Inc. All Rights Reserved. BUGBYTES, BUGS and the Bug logo are trademarks of BUGBYTES, Inc. All other product names are trademarks, registered trademarks, or service marks of their respective owners.
Last Updated October 1, 2001